ERMI Test
The Environmental Relative Moldiness Index
What Is ERMI?
The Environmental Relative Moldiness Index (ERMI) is an indoor air quality testing method developed by the U.S. Environmental Protection Agency (EPA) together with the U.S. Department of Housing and Urban Development (HUD). In essence, ERMI was designed to be a sensitive and standardized method to assess air quality and mold contamination. ERMI uses DNA-based technology to differentiate and quantify certain indoor mold species.
Furthermore, the test also statistically compares the results from your household to results from one thousand U.S. households, gathered from a national home survey. This then provides you with the ERMI rating of your home. Since it had been implemented in 2007, ERMI metrics have been used in several studies as an indicator and a predictor of mold contamination, moisture damages, and potential respiratory conditions.
ERMI vs. Visual inspections
Screening for molds and mold contamination in households can be done in many ways. When done by experts, visual observations can be a reliable method, especially when visual assessment is supported by microbial verification and performed by an experienced building inspector or engineer. Unfortunately, not all visual inspections can be performed by appropriately qualified personnel. Furthermore, mold contaminations can often be hidden inside structures.
ERMI vs. Air sampling
Another method for assessing possible indoor mold damage is air sampling. This is probably is the most commonly used method for quantifying indoor microbial species. The test is done by exposing special vacuum-pumped cartridges to air in several rooms in your home, usually 5 minutes or less. The trapped mold particles are then prepared and analyzed. The drawback of this system is the accuracy because short-term exposure to the indoor air might not be enough to access the air quality fully. You might say sort of like comparing a snapshot to a video. The limited reproducibility, particularly considering short-term air samples, might also be a downside.
ERMI vs. HERTSMI
The HERTSMI-2 test (an acronym for Health Effects Roster of Type-Specific Formers of Mycotoxins and Inflammagens – 2nd Version) is a smaller, more targeted indoor mold test that only searches for five particular mold species. These species can be connected with more serious respiratory conditions and are most commonly involved in Chronic Inflammatory Response Syndrome (CIRS). HERTSMI-2 is a derivative test of the ERMI, and although it might be cheaper, it is less robust because it searches for only five types of molds. This means that even though HERTSMI-2 uses similar technology, it is not as accurate in assessing indoor air quality as ERMI.
ERMI vs Tape sampling
Tape sampling is among the most commonly used techniques to test surfaces during mold inspections. Sampling is performed using packaged kit specifically designed for tape mold sampling, but it can also be performed using standard, clear and clean cellophane tape. The tape is pressed against a visible and moldy surface and is then sent for laboratory analysis. This method is non-invasive and non-damage to materials or surfaces. The sampling is also not complicated to perform. However, its drawback is that you can only perform this on visible mold colonies, while some light-spored, airborne genera may be easy to miss. Furthermore, not all mold spores settle onto flat surfaces easily. Because of these disadvantages and the fact that the sampling method covers only a small representative sample, the tape sampling method might be better suited only for limited qualitative and not quantitative mold analyses.
Concerned About Mold? Get a Free Virtual Inspection!
Worried about mold affecting your indoor air quality? Contact Mold Busters for a free virtual mold inspection. Our certified experts will provide a comprehensive assessment of your home to identify any mold issues, ensuring a safe and healthy environment for you and your family. Don’t wait—act now to safeguard your home!
The science behind ERMI
The ERMI test uses mold-specific quantitative polymerase chain reaction (MSqPCR) technology to (first) determine which of the 36 mold species may exist in your home and (second) quantify them. The tested samples come from the dust collected from your household. Settled dust represents an excellent repository of the overall microbial condition of a home. Therefore, this method is fairly accurate because certain surfaces, such as rugs, carpeting, or wooden floors, capture mold microflora particles due to the long-term propagule (spore) precipitation.

The sampled mold particles first need to be “opened” and their DNA extracted in the laboratory. This DNA is then amplified many thousand times (qPCR technology), so there are enough copies of certain genes for experts (or machines) to read and detect exactly which mold species are present. The qPCR technology also allows to approximately quantify (in real-time) the number of certain mold species present in your home. This can provide an insight into possible critical mold damage located somewhere in your home.
ERMI molds
The MSqPCR technology is homed to recognize one of the 36 mold species divided into two categories. The first category (Category 1) contains 26 mold species typically found at higher levels in water-damaged homes. The second category (Category 2) contains 10 common species not associated with water damages (common indoor molds). These 36 species have been chosen among the 82 species analyzed in samples of water-damaged and control homes. The quantified result can then be compared to a national database. This database was composed of results gathered from 1.096 households across the United States during the 2006 Housing and Urban Development (HUD) American Healthy Home Survey. Individual results, ranked from lowest to highest, were used to compile the national Relative Moldiness Index (RMI) Scale (Fig. 2).
ERMI Molds Table – First Category (Common in Water-Damaged Homes)
| Name | Ecology & habitat | Humans and Animals Pathogenicity | Plant Pathogenicity | Toxins | Importance |
|---|---|---|---|---|---|
| Aspergillus flavus | Soil and decaying wood | Invasive and non-invasive Aspergillosis; Impaired food consumption, stunted growth, immune suppression, and possible liver cancer development | Opportunistic pathogen of oil-containing crops such as maize, tree nuts, peanut seed and cottonseed | Aflatoxin B1 and B2 | None |
| Aspergillus fumigatus | Saprophytic (decaying organic and plant materials), opportunistic fungal pathogen | Invasive aspergillosis in immunocompromised humans; allergen | Opportunistic, non-specific phytopathogenicity | Fumitremorgens, verruculogen and gliotoxin | None |
| Aspergillus niger | Soil and decaying organic matter | Non-pathogenic to humans (unless in cases of severely immunocompromised persons) | None | None | Important in food industry for production of citric acid. Also pectinase, protease and amyloglucosidase |
| Aspergillus ochraceus | Soil fungus and contaminant of agricultural commodities | As a contaminant can cause neurotoxic, immunosuppressive, genotoxic, carcinogenic and teratogenic effects | None | Ochratoxin A, citrinin, mellein | None |
| Aspergillus penicillioides | Airborne contaminant, common on dry materials | Associated with allergic rhinitis, facilitates the growth of house dust mites | None | None | Potential for treatment of petrochemical effluents |
| Aspergillus restrictus | Low water requirement, common colonizer of stored commodities | Non-pathogenic, possibly and allergen | None | None | None |
| Aspergillus sclerotiorum | Soil and stored goods | None | None | None | Potential in termite and plant pathogen control |
| Aspergillus sydowii | Saprophyte found in soil and plant matter | Aspergillosis, onychomycosis, and keratomycosis in humans; Coral reef pathogen | None | Indole alkaloids | None |
| Aspergillus unguis | Soil and decomposing plant matter and moist substrates such as building materials | Frequently found the homes of asthmatic children, can cause superficial mycosis | None | Sterigmatocystin | None |
| Aspergillus versicolor | Soil, plant debris, marine environments, indoor, food contaminant | Opportunistic pathogen, can cause aspergillosis and onychomycosis | None | Sterigmatocystin and cyclopiaxonic acid | Potential for heavy metal removal, enzyme purification |
| Aureobasidium pullulans | Generalist species (soil, air), epiphyte and endophyte on many plant species | Contaminant in humidifiers or air conditioners, causes humidifiers or air conditioners (“humidifier lung”). | None | None | Potential for enzyme production (amylase, proteinase, lipase, cellulase, xylanase, manganese, transferases) |
| Chaetomium globosum | Saprophytic on plants, soil, straw, and dung. Also indoors and on wooden items | As an indoor contaminant can cause non-atopic asthma, sinusitis, and respiratory problems. Also causes onychomycosis and cutaneous infections. | None | Emodins, chrysophanol, chaetoglobosins A and C, chetomin | None |
| Cladosporium sphaerospermum | Saprophytic species, opportunistic plant pathogen | Allergen in humans with respiratory problems; subcutaneous phaeohyphomycosis | Secondary parasite on weakened and dying plants, biofertilizer | Radiotrophic fungus (ability to use radiation as an energy source to stimulate growth) | None |
| Eurotium chevalieri | Saprophytic, xerophilic species | Opportunistic pathogen that causes human cutaneous aspergillosis | None | Gliotoxin, Sterigmatocystin | None |
| Paecilomyces variotii | Thermophilic, Common saprophyte (composts, soils, food), Indoor mold | Generally non-threatening; Occasional reports of respiratory problems in immunocompromised individuals | None | None | None |
| Penicillium brevicompactum | Saprophytic species, frequent fruit inhabitant, occasional spoilage of stored goods | None | None | None | Source of Mycophenolic acid (immunosuppressant drug) |
| Penicillium corylophilum | Common soil species, psychrophilic and xerophilic | Potential respiratory irritant | None | Citrinin, epoxyagroclavine | Pathogenic to mosquitoes |
| Penicillium crustosum | Spoilage of protein-rich foods | Consumation may cause transient neurological problems (convulsion, tremors, ataxia, tachycardia) | None | Penitrems A through G, thomitrems A and E, roquefortine C | None |
| Penicillium purpurogenum | Saprophytic species, soil and plant matter | Rarely causing respiratory issues | None | None | Produces lovastatin |
| Penicillium spinulosum | Cosmopolitan saprophyte, thrives in low temperatures and low water availability | Unlikely to cause human infection as it can grow above 37 °C | None | None | None |
| Penicillium variabile | Cosmopolitan endophyte, saprophyte | None | None | None | Potential use in biocontrol of vegetable pathogens |
| Scopulariopsis brevicaulis | Saprotroph, various organic matter | Drug resistant, opportunistic pathogen that causes skin-related diseases | None | Satratoxin H, atranones A trough G | None |
| Stachybotrys chartarum | Saprophytic, often found on cellulose-rich building materials in damp environment | Respiratory, dermatological, eye and constitutional problems | None | None | None |
| Trichoderma viride | Saprophytic and parasitic on other fungi | None | None | None | Useful for biocontrol of plant pathogens |
| Wallemia sebi | Indoor mold, xerophilic and often found on highly sugared or salted products | Respiratory inflammations, allergenic | None | Wallimidione, walleminol, walleminone | None |
ERMI Molds Table – Second Category (Not Common in Water-Damaged Homes)
| Name | Ecology & habitat | Humans and Animals Pathogenicity | Plant Pathogenicity | Toxins | Importance |
|---|---|---|---|---|---|
| Acremonium strictum | Saprotroph found in soil, plant debris, and rotting mushrooms | Causes rashs and flu-like problems. Respiratory infections in immunocompromised individuals. | Plant pathogen and plant endophyte | None | Potential in biocontrol of Silver Scurf potato disease |
| Alternaria alternata | Plant pathogen, indoor mold | Associated with active asthma symptoms | Causal agent of leaf spot in a high number of plants | None | None |
| Aspergillus ustus | Indoor and soil mold | Isolated cases of human onychoses | None | None | None |
| Cladosporium cladosporioides | Xerophilic, xerotolerant, and a psychrophilic plant facultative parasite | Rarely and predominantly superficial infections. Phaeohyphomycosis and respiratory problems in immunocompromised individuals | Causes fruit rot and blossom blight of berries | None | None |
| Cladosporium herbarum | Common saprophyte in soil and organic debris, psychrotrophic | Allergen, causes asthma and hay fever | None | None | Frequent contaminant of refregerated meats and dairy products |
| Epicoccum nigrum | Pathogen of plants and other fungi, endophyte | Associated with allergic asthma, rhinitis, hypersensitivity pneumonitis, and allergic fungal sinusitis | Leaf Spot Disease | None | Pigment and nanoparticles production, bio-control of crop pathogens |
| Mucor racemosus | Dimorphic plant saprophyte, opportunistic human pathogen | Pulmonary, cutaneous, and gastrointestinal infections in immunocompromised individuals | None | None | Soy fermentation, production of industrial enzymes, potential in production of anticancer compounds |
| Penicillium chrysogenum | Often indoor (damp or water-damaged) mold | Skin, ear, sinus infections, pneumonia in immunocompromised individuals | None | None | The original source of penicillin |
| Rhizopus stolonifer | Common saprophyte, stored foods decomposer | Opportunistic agent, causes Mucormycosis in immunocompromised individuals | Damages ripe fruit | None | None |
How to do an ERMI test?
The practicality of the ERMI test lies in that you can conduct the sampling of your home. The samples are collected by vacuuming a standard size area of the floor in several rooms of your home. This is done with a special vacuum cleaner extension nozzle and a dust collector, provided with the ordered ERMI mold test kit. Vacuuming is done on a 1m x 2m (~39 x 79 inches) rectangular sampling area for 5 minutes. The dust collector needs to be placed into a re-sealable bag and sent to an expert laboratory via mail. If your home doesn’t have carpeting, the samples can be collected via special Swiffer cloths.
Interpreting ERMI test results
Based on the results found in your home, mold species are selected and grouped into those with high concentrations in moldy homes (Group 1) and those with low concentrations (Group 2). To calculate your ERMI index, the individual concentrations of the mold species detected in your home are statistically transformed into index values, and the Group 2 value is subtracted from Group 1. This is your ERMI value, which can range between -10 and +20, and is interpreted accordingly (Fig. 2):
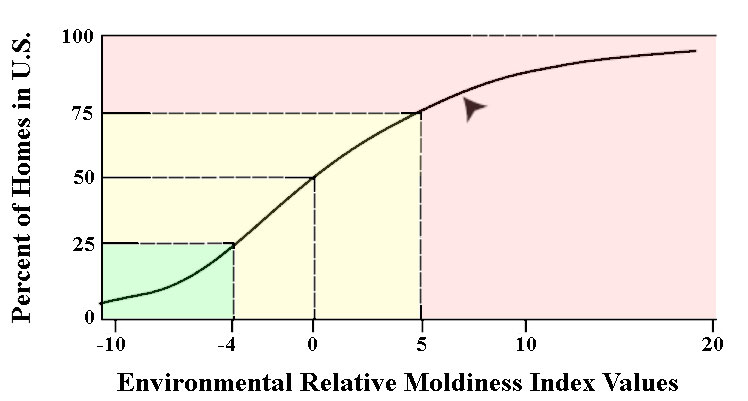
- Level 1: ERMI value ≤-4 represent a low relative mold index, and mold contamination is unlikely;
- Level 2 to Level 3: ERMI value >-4 to 0 and > 0 to ≤ +5 represent a moderately high index, and further investigation should be conducted to establish if your home has a mold contamination problem;
- Level 4: ERMI value >+5 to +20 represents a high relative moldiness, and further investigation is needed to determine the source of mold contamination.
References:
- Vesper, S., McKinstry, C., Haugland, R., Wymer, L., Bradham, K., Ashley, P., … & Friedman, W. (2007). Development of an environmental relative moldiness index for U.S. homes. Journal of Occupational and Environmental Medicine, 49(8), 829-833.
- Vesper, S., Wakefield, J., Ashley, P., Cox, D., Dewalt, G., & Friedman, W. (2011). Geographic distribution of Environmental Relative Moldiness Index molds in USA homes. Journal of environmental and public health, 2011.
- Vesper, S. J., Wymer, L., Kennedy, S., & Grimsley, L. F. (2013). Decreased pulmonary function measured in children exposed to high environmental relative moldiness index homes. The open respiratory medicine journal, 7, 83.
- Vesper, S., & Wymer, L. (2016). The relationship between environmental relative moldiness index values and asthma. International journal of hygiene and environmental health, 219(3), 233-238.
- Täubel, M., Karvonen, A. M., Reponen, T., Hyvärinen, A., Vesper, S., & Pekkanen, J. (2016). Application of the environmental relative moldiness index in Finland. Applied and environmental microbiology, 82(2), 578-584.

Get Special Gift: Industry-Standard Mold Removal Guidelines
Download the industry-standard guidelines that Mold Busters use in their own mold removal services, including news, tips and special offers:
"*" indicates required fields
Published: August 16, 2021 Updated: July 19, 2024

Written by:
Dusan Sadikovic
Mycologist - MSc, PhD
Mold Busters
Fact checked by:
Michael Golubev
CEO
Mold Busters
